Detail
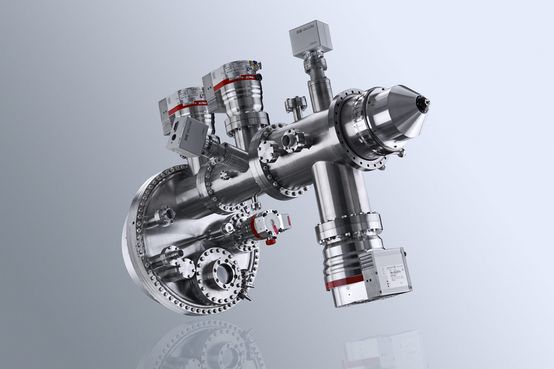
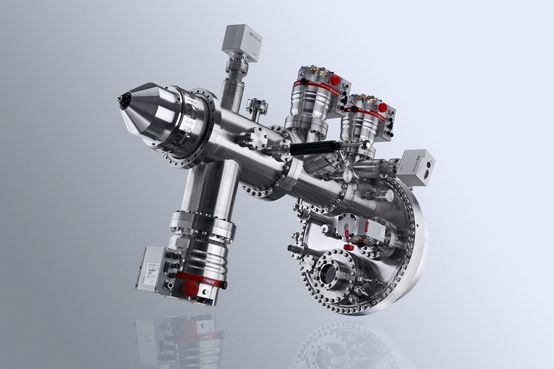
DetailPHOIBOS 150 NAP 1D-DLD
State of the art hemispherical energy analyzer with 1D-DLD detector for photoelectron spectroscopy measurements (XPS and UPS) in the pressure regime from UHV to near ambient pressure (NAP)
The PHOIBOS 150 NAP Analyzer is a true 180° hemispherical energy analyzer with 150 mm mean radius. It consists of a differentially pumped electrostatic pre-lens, with a three-stage differentially pumped PHOIBOS 150 analyzer. Thus, the design concept provides four separate pressure stages separated by apertures. The first pumping stage (pre-lens) is separated from the analytic chamber by a nozzle with a customizable opening at the tip with diameters between 0.3 mm and 1 mm. By using a turbopump on the pre-lens stage, a pressure difference of four orders of magnitude (compared to the analysis chamber) can be achieved.The first and second stages are separated by an aperture. An in-lens gate valve allows a high-vacuum seal between of the PHOIBOS 150 Analyzer and NAP pre-lens and enables venting of the analysis chamber and pre-lens without venting the energy analyzer.
For Imaging NAP-XPS the pre-lens can be equipped with the SPECS NAP-XPS Imaging Lens Module that supports two different operation modes, one optimized for lateral resolution and one for transmission. In the lateral resolving mode the acceptance angle can be freely adjusted between +/- 3° to +/- 8° giving an ultimate lateral resolution of better than 10 µm with an acceptance area of 0.6 mm in diameter.
KEY FEATURES
- Wide Angle Pre-Lens with 44 ° Acceptance Angle
- Near Ambient Working Pressures up to 100 mbar (depending on configuration)
- Large pass energy range
- Working range up to 3.5 keV (upgradable to 7 keV with corresponding power supply and detector)
- High energy and angular resolution
- High spatial resolution with imaging lens module
- Ultimate flexibility by switching between high transmission and high lateral resolution mode
- Fast detectors with high dynamic range for fast real time data acquisition in snapshot mode
- Pneumatic in-lens gate valve
- 4 differential pumping stages
- Full detector flexibility (MCD, 1D-DLD, 2D-DLD, 2D-CCD/-CMOS)
- MADE FOR THESE METHODS
- SPECIFICATIONS
- RELATED PRODUCTS
- APPLICATION NOTES
- PUBLICATIONS
- SPARE PARTS
- DOWNLOADS

R&D100 in 2010
The PHOIBOS 150 NAP Analyzer won the R&D 100 award for the best 100 products developed in 2010.
SPECIFICATIONS
| Mounting Flange | DN150 CF (8" OD) |
| Working Distance | 300 - 500 µm (for standard nozzle with 300 µm diameter) |
| Energy Dispersion | Hemisphere |
| Slits/Apertures | 8 Entrance, 3 Exit slits and Iris aperture |
| Magnetic Shielding | Double µ-Metal Shielding |
| Lens Modes | Angular Resolving and Transmission Lens Modes |
| Kinetic Energy Range | 0 - 3500 eV |
| Pass Energies | 0 - 550 eV continously adjustable |
| Detector | 1D-DLD Detector |
| Measurement Modes | Snapshot mode, Sweeping mode, Fixed energy mode |
| Energy Window | 13% of Pass Energy |
| Electric Isolation | up to 7 keV |
| Electronics | HSA 3500 plus HT 100 for analyzer and HSA 3500 plus HT 173 for pre-lens |
| Working Pressure | up to 30 mbar (higher pressure values achievable with corresponding nozzle diameters and differential pumping packages) |
| Acceptance Angle | ±22° |
| Angular Resolution | only with 2D detector |
| Energy Resolution | < 10 meV |
| Lateral Resolution | only with 2D detector |
| Smallest Acceptance Spot | Without imaging lens module the smallest acceptance spot is defined by the excitation spot of X-ray source or nozzle diameter |
| Detector Channels | Max. 960 energy channels (240 and 120 channels for binning 4 and 8, respectively) |
| XPS Count Rates UHV | 70 kcps (guaranteed), 150 kcps (achievable) * |
| XPS Count Rates 10 mbar | 7 kcps (guaranteed), 15 kcps (achievable) * |
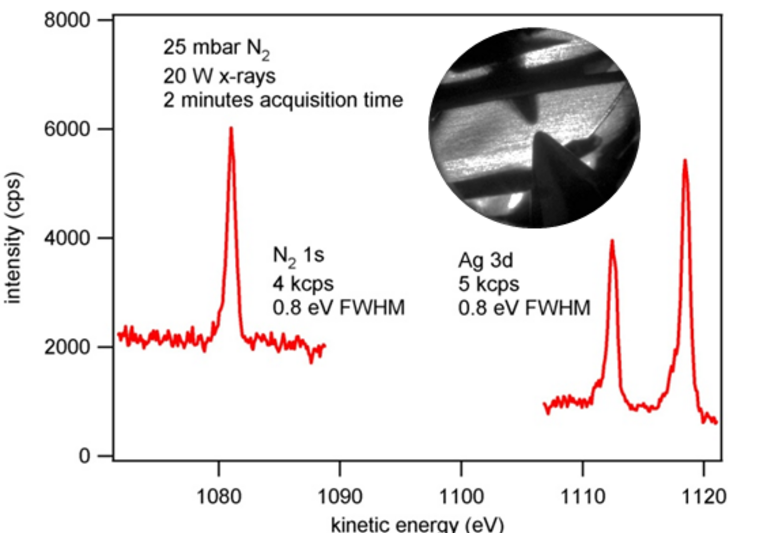
| XPS Count Rates 25 mbar | 0.5 kcps (guaranteed), 1.5 kcps (achievable) * |
| . | * Ag 3d, FWHM < 0.85 eV, small spot monochromated X-ray source µ-FOCUS 600, Al Kα anode, 20 W, Spot size < 250 µm |
SPARE PARTS
Spare electrical feedthrough for all DLD detectors. Connection flange for the ACU unit
Nozzle 0,3 mm for 7 kV PHOIBOS 150 Backfilling Pre-Lens

Nozzle 1,0 mm for 7 kV PHOIBOS 150 Backfilling Pre-Lens

Replacement feedthrough for PHOIBOS Release R5 & R6 iris mechanism

Replacement spindle for PHOIBOS Release R5 & R6 iris mechanism