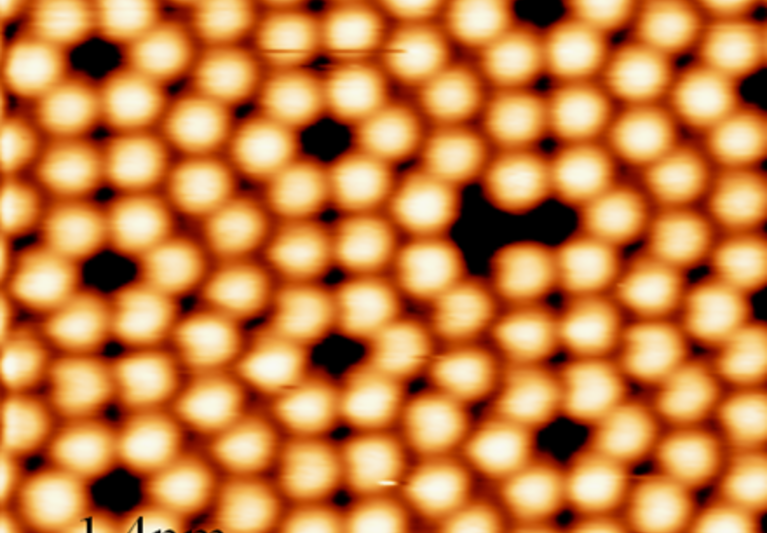
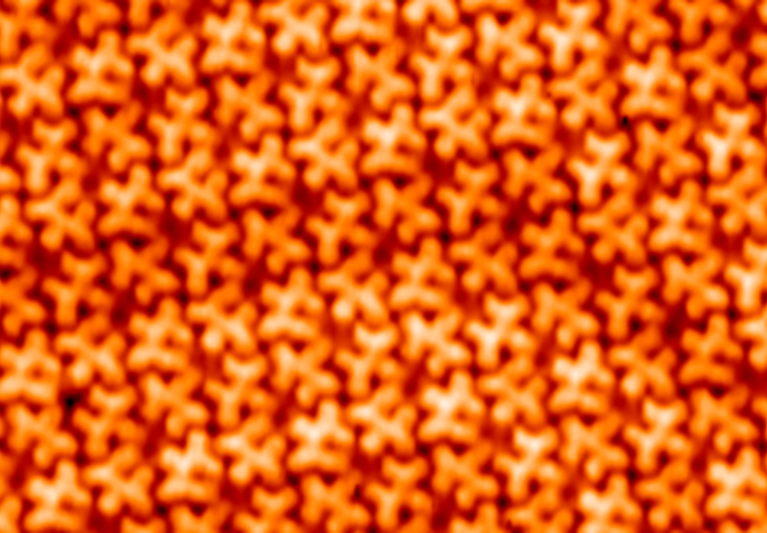
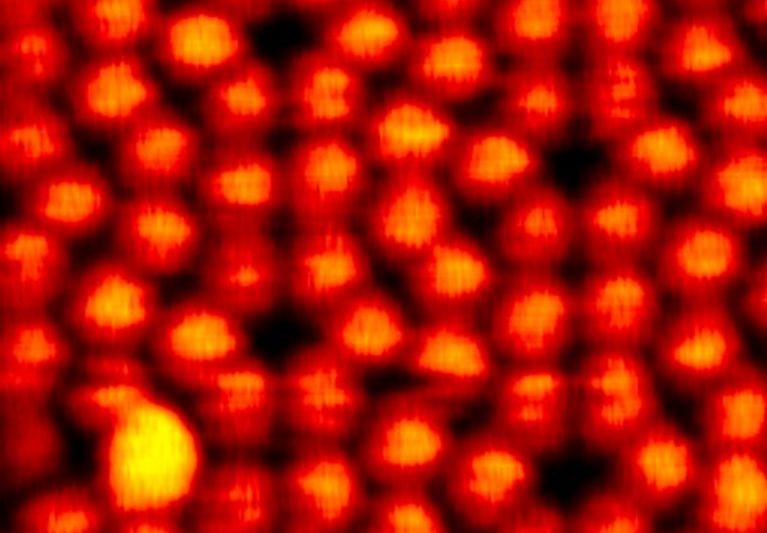
Chemical reactions that convert sp2 to sp3 hybridization have been demonstrated to be a fascinating yet challenging route
to functionalize graphene. So far it has not been possible to precisely control the reaction sites nor their lateral order at the
atomic/molecular scale. The application prospects have been limited for reactions that require long soaking, heating, electric
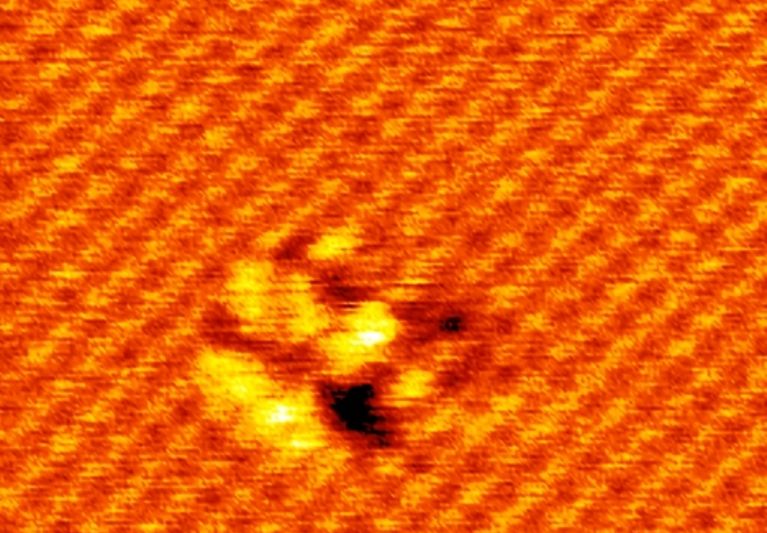
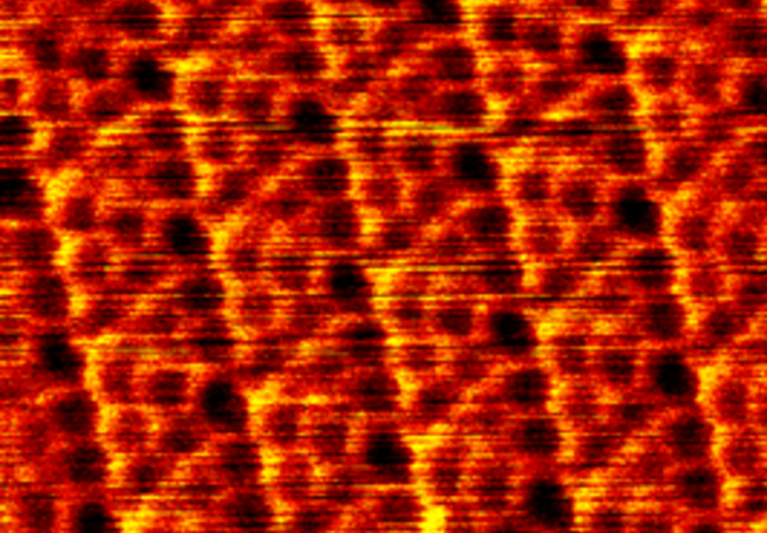
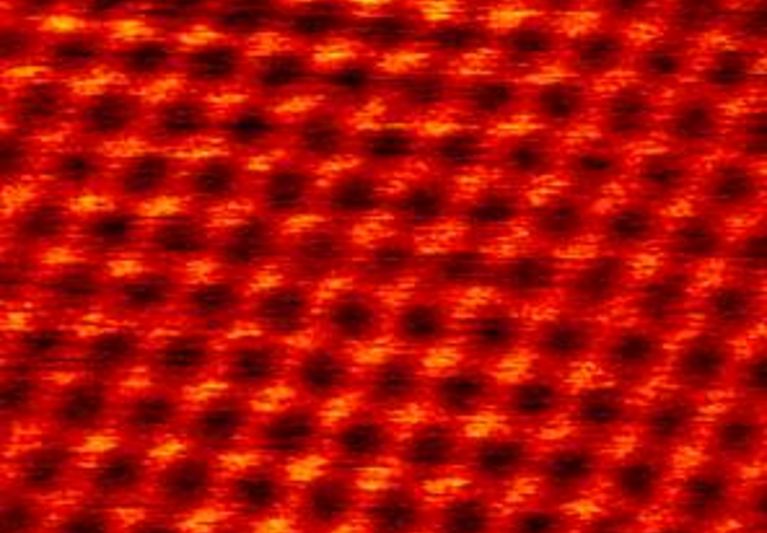
pulses or probe-tip press. Here we demonstrate a spatially selective photocycloaddition reaction of a two-dimensional
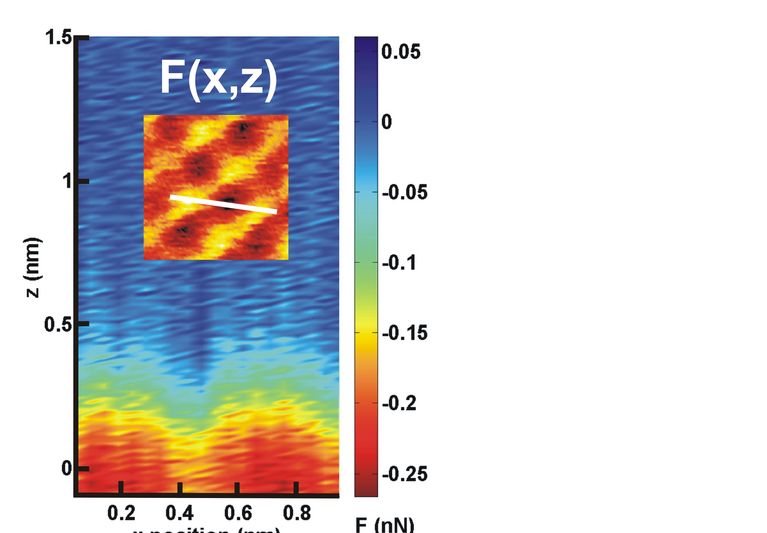
molecular network with defect-free basal plane of single-layer graphene. Directly visualized at the submolecular level, the
cycloaddition is triggered by ultraviolet irradiation in ultrahigh vacuum, requiring no aid of the graphene Moiré pattern. The
reaction involves both [2+2] and [2+4] cycloadditions, with the reaction sites aligned into a two-dimensional extended and
well-ordered array, inducing a bandgap for the reacted graphene layer. This work provides a solid base for designing and engineering
graphene-based optoelectronic and microelectronic devices.
M. Yu, C. Chen, Q. Liu, C. Mattioli, H. Sang, G.Shi,
W. Huang, K. Shen, Z. Li, P.Ding, P. Guan, S. Wang,
Y. Sun, J. Hu, A. Gourdon, L. Kantorovich, F. Besenbacher,
M. Chen, F. Song and F.Rosei
Nature Chemistry volume 12, pages1035–1041 (2020)