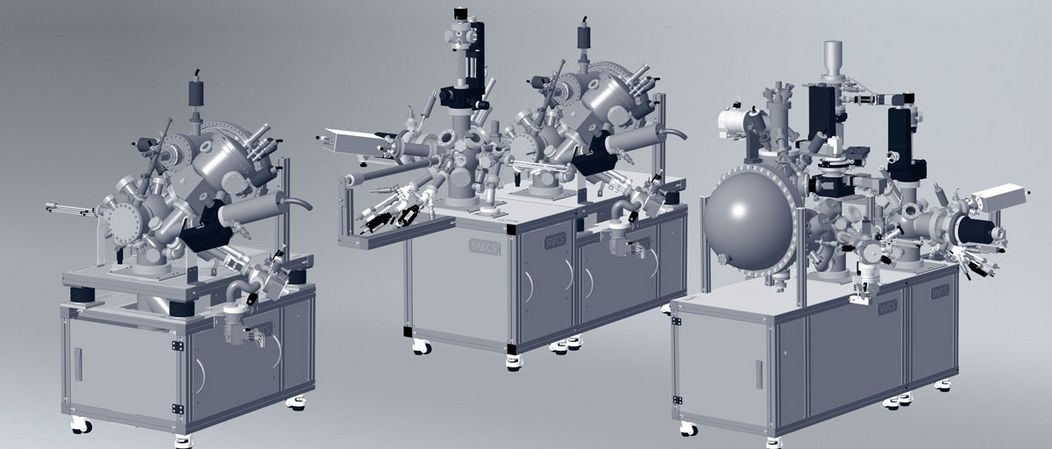
The product portfolio of SPECS is enlarged with the ProvenX (proven eXperience) System Series which represents the quintessence of our extensive knowledge in developing and manufacturing complete systems for surface analysis that fulfills the most demanding scientific requirements. The series contains dedicated systems for ARPES, µ-ARPES and Momentum Microscopy, XPS/UPS as well as NAP-XPS.
The basic concept lying underneath is having a fixed, compact system layout and a large flexibility in choosing the analytical components like hemispherical electron energy analyzer, X-ray source, UV source, ion source, flood gun, etc. All possible components and system configurations have been already proven and found working. Since the ProvenX system series is a summing-up of all systems that we built up to now, the performance is confirmed and therefore, the system specifications, system drawings, factory and site acceptance tests as well as installation requirements are already available. The ProvenX systems are ready-to-order and come with a short delivery time from date of order.